Abstract
Background: Although bronchiolitis obliterans syndrome (BOS) is a well described manifestation of pulmonary involvement by chronic graft-versus-host disease (PcGvHD), additional pulmonary phenotypes of PcGvHD beyond BOS have not been well-characterized. PcGvHD and chronic lung allograft dysfunction (CLAD) in lung transplant recipients share similar pathophysiological mechanisms. We aim to use an adaptation of the International Society for Heart and Lung Transplantation (ISHLT) CLAD Consensus Criteria to describe PcGvHD phenotypes beyond BOS in order to improve the diagnosis and classification of PcGvHD.
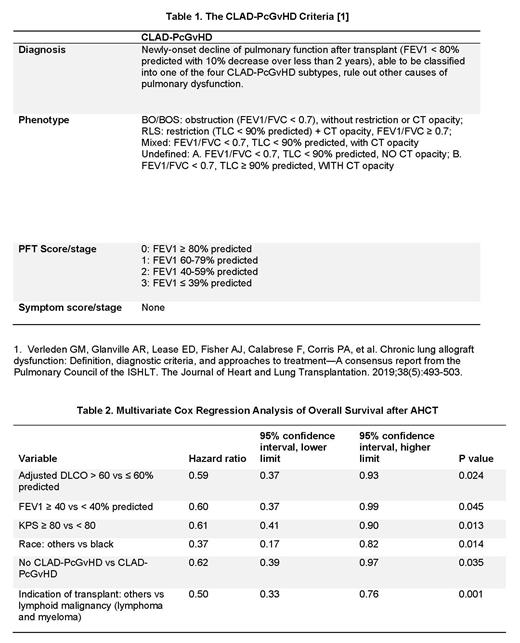
Methods: We created a new PcGvHD diagnostic and phenotyping criteria based on the 2019 ISHLT CLAD Consensus Criteria (Table 1). Consecutive patients enrolled in the cross-sectional National Institutes of Health (NIH) natural history protocol (NCT00092235) between October 2004 and January 2020 were analyzed for study inclusion. Diagnosis of CLAD-PcGvHD was made when the following criteria were met: 1). New-onset decline of pulmonary function after allogeneic stem cell transplant (AHCT); 2). Forced expiratory volume in one second (FEV1) < 80% predicted with 10% decrease over less than 2 years; 3). Classification into one of the four CLAD-PcGvHD subtypes, [BOS, restrictive lung syndrome (RLS), mixed BOS/RLS (M) and undefined (UD)] was possible; 4). Other causes of pulmonary dysfunction were excluded. Patients without a diagnosis of cGvHD, NIH-performed pulmonary function test (PFT) or thoracic computed tomography (CT) were excluded. Demographic characteristics, AHCT information, GvHD history and treatment information, PFTs, and CT were collected for all study cohort patients. PFTs were analyzed by two independent pulmonologists and thoracic CT studies by an independent cardiothoracic radiologist. The final diagnosis and classification of PcGvHD per the new criteria were made and confirmed by two independent reviewers.
Results: Of a total of 447 patients, 21 patients did not have cGvHD, 76 patients did not have NIH-performed PFT and/or CT, leaving 350 patients for the study cohort. The NIH cGvHD global severity score was 3 (severe) in 76% of patients. Evaluation of the study cohort by the new criteria diagnosed CLAD-PcGvHD in a total of 166 (47.4%) patients, including: 12 (3.4%) BOS, 67 (19.1%) RLS, 47 (13.4%) mixed, and 40 (11.4%) UD. In contrast, only 80 (22.9%) patients had documented diagnosis of BOS according to the NIH 2014 cGvHD consensus criteria, i.e., FEV1 < 75% predicted and FEV1/FVC < 0.7. An additional 52 (14.9%) patients had reduced FEV1, but were unable to be classified by the CLAD-PcGvHD criteria (unclassified, UC), therefore did not meet the diagnosis of Pc-GvHD. Compared to patients with normal FEV1 or UC, patients with CLAD-PcGvHD had poorer Karnofsky performance status (KPS) (p=.0001), more commonly received myeloablative conditioning (p=.047) or busulfan (p=.007), had a higher prevalence of liver cGvHD (p=.001), and required more lines of therapy for cGvHD (p=.0001). Between different CLAD-PcGvHD phenotypes, patients with BOS were older at AHCT (p=.005), had higher FEV1 and hemoglobin-adjusted DLCO (p=.0001 and p=.005, respectively), while patients with RLS had higher frequencies of skin or joint/fascia cGvHD (p=0.001 and p=.003, respectively). Median overall survival (OS) after AHCT was 162 months in patients with CLAD-PcGvHD, unreached in UC, and 310 months in patients with normal FEV1 (p=.007); OS was similar between different PcGvHD phenotypes (p=.154). In multivariate analysis, KPS < 80, FEV1 < 40%, adjusted DLCO < 60%, CLAD-PcGvHD, indication for AHCT being lymphoid malignancy, and black race were independent risk factors for OS (Table 2).
Conclusion: The CLAD-PcGvHD criteria involves computed tomography in patient evaluation and encompasses a wide spectrum of lung disease post-AHCT. With this criteria, half of the patients in the NIH cGVHD natural history study could be diagnosed and phenotypically classified. The development of CLAD-PcGvHD was an independent risk factor for post-transplant survival. The proposed criteria could become a valuable all-encompassing clinical tool in studying post-AHCT lung disease and facilitate the study across solid organ and hematopoietic stem cell transplantation.
Pavletic: Center for Cancer Research: Research Funding; National Cancer Institute: Research Funding; National Institutes of Health: Research Funding; Celgene: Research Funding; Actelion: Research Funding; Eli Lilly: Research Funding; Pharmacyclics: Research Funding; Kadmon: Research Funding.